Professor Kazem Fathie, M.D., F.A.C.S., F.I.C.S.,Ph.D.
Introduction
The Departments of Pathology at the Mayo Clinic and Columbia University have agreed as to the, histological diagnosis of the unusual tumor we are reporting, namely, a hemangiopericytoma of the thoracic spine.
Case Report
This 21-year-old white man was first seen on August 31, 1967, because of progressive difficulty in walking, urinary incontinence, and pain in the chest wall and in both legs. Just before admission the patient not only had a tendency to fall but was barely able to get up. The rest of his family and past history was irrelevant except for 2 years of recognized hypertension.
Examination.- General physical examination, except for the blood pressure of 170/80, was entirely normal. The deep tendon reflexes of the legs were exaggerated, more so on the left. There was a positive sustained left ankle clonus and the Babinski was positive bilaterally. The Hoffman and Rhomberg signs were normal as were perception of vibration, light touch, and deep pressure. The patient tended to drag the left leg and was unable to walk on tip toes or on his heels. There was no muscular atrophy; muscle tone in the legs was increased. He could not get up from the sitting position without help. There was a questionable sensory abnormality along the nipple line. Ophthalmological and cranial nerve examinations were normal. Electroencephalography, brain scan, x-ray examination of the total spine and skull, and electrocardiogram were normal. Myelography showed a complete block at T-6.
Operation. Laminectomy of T-4 through T-7 was carried out on September 11, 1967. At T-6 there was a rather hard extradural tumor measuring 21/2 X 1cm. This encapsulated, elliptical, pinkish gray tumor was adherent to the dura and was removed in one piece.
Histological Examination. Studies showed a poorly differentiated neoplasm consistent with the diagnosis of hemangiopericytoma. There was some nuclear variability and hyperchromatosis with moderate to mild mitotic activity (Fig. 1). Special stains showed prominent reticular architecture (Fig. 2) with cellular areas suggesting alignment of the cells around the vessels, but this was not in a very uniform or unequivocal pattern. There was also a poorly preserved fragment of fibrofatty stroma with foci of polygonal and fusiform cells, and a mixture of lymphocytes and scattered polymorphonuclear cells (Fig. 1).
Postoperative Course. The patient’s neurological status rapidly returned to normal. Function of the bladder, bowel, as well as walking, ability to stand up, and strength in both legs all were normal within 2 weeks after surgery. The left ankle clonus and bilateral Babinski disappeared within 10 days of operation. There has been no sign of return of these abnormal signs.
Discussion The specimen was studied at the Mayo Clinic by Dr. E. H. Soule who described the tissue as follows: “The single hematoxylin-and-eosin-stained tissue section submitted for examination revealed a neoplasm that is composed of rather uniform, closely packed, plump spindle cells. These cells exhibit little pleomorphism and only an occasional mitotic figure. There are numerous dilated thin-walled vascular spaces throughout the section. Close inspection reveals many fine capillary-like vessels some of which appear to be compressed by the neoplastic cells. A reticulin stain would undoubtedly reveal more of these occult capillaries and demonstrate a fine reticulin network about individual tumor cells. The finding of reticulin would exclude the origin of the neoplastic cells from endothelium. The histologic pattern is that of a hernangiopericytoma of a lower order of malignancy.”
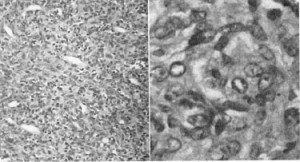
Fig,. 1. Left: Low-power photomicrograph showing compact area of poorly differentiated neoplasm with nuclear variability and hyperchromatosis as well as mitotic figures. Note the reticular architecture and fibrofatty stroma with foci of polygonal and fusiform cells with a mixture of lymphocytes and scattered polymorphonuclear cells. H. & E., XIOO. Right: High-power photomicrograph of hemingio- pericytoma demonstrating mitotic cells, pleomorphism, dilated thin vessel cells varying from fusiform to spindle-shaped and oval, containing vesicular nuclei, with hyperchromatosis. H. & E., X400.
Neuropathologists at Columbia University agreed with the diagnosis, and were impressed by the reticulin stain which showed occasional areas of “epithelial” appearance, that is, areas in which there are sheets of tumor cells with little or no reticulin. Dr. Raffaele Lattes pointed out: “these vascular meningeal tumors have been frequently classified as hemangiopericytoma even though they vary in one way or another from the more classical hemangiopericytoma of the soft tissues.”
Dr. K. B. Grant reported: “Sections show a fibrocollagenous, partially hyalinized periphery of capsular-like tissue enclosing an extremely cellular compact tumor composed of innumerable vascular spaces with the proliferating cells varying from fusiform and spindle to slightly oval and containing vesicular nuclei. In occasional foci at the border, the tumor appears to interrupt the capsule. Nuclear variability and hyperchromatosis is variable. There is a moderate to mild mitotic activity but no atypical figures are seen. in some cells nucleoli are moderately prominent. Special stains show a prominent reticular architecture with the very cellular areas suggesting alignment of cells around vessels, but this is not a very uniform or unequivocal pattern. There is a preserved fragment of fibrofatty stroma with foci of polygonal and fusiform some suggesting those seen in the first specimen. Additionally there is an intimate admixture of lymphocytes and scattered polymorphs with some RBC extravasation.”
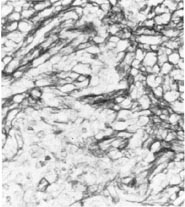
Fig. 2. Reticulin stain demonstrates prominent reticular architecture and fibrofatty stroma with foci of polygonal and fusiform cells with capillaries and a fine reticulin network about the individual tumor cells. Reticulin, X400.
Different authors have mentioned hemingiopericytorna in the pelvis(21), soft tissue(23), muscles(23), trunk(17), thigh (24), Orbit(10), dura(16) thorax(8 ), thoracic aorta(2), retroperitoneal(5), nasal cavity(13), stomach(6), oral cavity(4), perirenal(20), kidney(7), omentum(12), uterus(14), mouth and jaw(9), and urachus(1). Spiro, et al.(21) in 1964 stated that 26% of the cases show incidence of metastases, and local recurrence has been listed at 25%. O’Brien and Brasfield(17) reported 24 patients with hemangiopericytoma; these included 14 females and 10 males who were between 20 and 68 years of age. The most common location in this series was the trunk. Seven were reported in the head and neck, two in the upper extremities, and nine in the trunk. The morbidity and mortality was 56.5%, which is considerably higher than those of other reports, which vary from 11.7% (Stout and Murray 22) to 45 % (Fisher 9).
The potentially malignant behavior of this tumor has been emphasized previously by Brown(3). Tissue cultures by Stout and Murray(22) in 1942 demonstrated that the cell of origin was the capillary pericyte of Zim- mermann. The pericyte, as described by Zimmermann(25) is a specific type of cell related closely to smooth muscle cell but having no contractile fibers. MacCarty and Brown(15) reported 40 cases of children with orbital tumors that were removed by transcranial approach, but found no hemangiopericytomas, although intraorbital hemangiopericytoma has been recorded previously. Ramsey(18), described the fine structures of hemangiopericytoma and hemangio-endothelioma and the fact that they are closely related; both consist of thin-walled vascular channels together with tumor cells in a network of extracelluar material. Three dural tumors were described by Muller and Mealey, Jr.(16), which were grossly indistinguishable from meningioma but with microscopic features of cerebellar angioblastoma, hence named “angioblastic meningioma.” Gensler, et al.,(11) described a giant benign hemangiopericytoma uniform functioning as an arteriovenous shunt. A pri- poorly mary thoracic hemangiopericytoma was described by Feldman and Seaman(8) who added 11 cases to a previous study and mentioned the recurrence of this tumor after many years. A pedunculated bemangiopericytoma at- tached to the thoracic aorta was reported by Blenkinsopp and Hobbs(2).
Summary
We have reported the successful removal of a hemangiopericytoma of the thoracic spine. The characteristic appearance of the tumor has been described and the diagnosis verified by three separate pathologists. Acknowledgments The author gratefully acknowledges the histological reports from K. B. Grant, M.D., St. Lukes Methodist Hospital, Cedar Rapids, Iowa; E. H. Soule, M.D., Mayo Clinic, Rochester, Minnesota; and Raffaele Lattes, M.D., Columbia University, New York.
References 1. Baglio, C. M., and CROWSON, C. N. Hemangiopericytoma of Urachus: report of a case. J. Urot., 1964, 91:660-662.
2. BLENKINSOPP, W. K., and HOBE, J. T. Pedunculated hemangiopericytoma attached to the thoracic aorta. Thorax, 1966, 21:193-196.
3. BROWN, D. N. Intracranial hemangiopericytoma-a clinical and pathologic study. (Thesis) Graduate School, University of Minnesota, 1962.
4. DAS, A. K., and GANS, B. J. Hemangiopericytoma of the oral cavity. Review of the literature and report of a case. J. Oral Surg., 1965, 23:456-460.
5. DOUGLAS, J. L., MITCHELL, R., and SHORT, D. W. Retroperitoneal hemangiopericytoma. Br. 1. Surg., 1966, 53:31-33.
6. ERNST, C. B., ADFLL, M. R., and KAHN, D. R. Malignant hemangiopericytoma of the stomach. Surgery, St. Louis, 1965, 58:351-356.
7. FARROW, G. M., HARRISON, E. G., JR., UTZ, D. C., and RFMINE, W. H. Sarcomas and sarcomatoid and mixed malignant tumors of the kidney in adults. Part 1. Cancer, N.Y., 1968, 22′:545-550.
8. FELDMAN, F., and SEAMAN, W. B. Primary thoracic hemangiopericytoma. Radiology, 1964, 82′:998-1008.
9. FISHER, J. H. Hemangiopericytoma: a review of 20 cases. Can. med. Ass. I., 1960, 83:1136-1139.
10. FUSTE, F. J. Primary hemangiopericytoma of the orbit. J. Tenn. med. Ass., 1968, 61:1003-1004.
11. GENSLER, S., CAPLAN, L. H., and LAUFMAN, H. Giant benign hemangiopericytoma functioning as an arteriovenous shunt. J. Am. med. Ass., 1966, 198:203-206.
12. GOLDBERGER, R. E., and SCHEIN, C. J. Hemangiopericytoma of the omentum: report of a case with a unique presentation and review of literature. Am. Surg., 1968, 34:291-295.
13. LENCZYK, J. M. Nasal hemangiopericytoma. Archs Otolar., 1968, 87:536-539.
14. LIERAS, H., VAUZFLLE, J. L., GUELPA, G., NERDHARDT, J. H., and TAiRRAz, J. P. Hemangiopericytoma of cystic form of uterus. Diagnostic and therapeutic problems. Lyon chir., 1965,61:863.
15. MACCARTY, C. S., and BROWN, D. N. Orbital tumors in children. Clin. Neurosurg., 1964, 11:76-93.
16. MULLER, J., and MEALEY, J., JR. Hemangiopericytema versus angioblastic meningioma. A tissue culture study. J. Neuropath. exp. Neurol., 1967, 26:140-141.
17. O’BEUEN, P., and BRASFIELD, R. D. Hemangiopericytoma. A follow up study. Cancer, N. Y., 1965, 18:249-252.
18. RAMSEY, H. J. Fine structure of hemangiopericytoma and hemangio-endothelioma. Cancer, N. Y., 1966,19:2005-2018.
19. SHKLAR, G., and MEYER, 1. Vascular tumors of the mouth and jaw. Oral Surg., 1965, 19:335-358.
20. SIMON, R., and GREENE, R. C. Perirenal hemangiopericytoma. A case associated with hypoglycaemia. J. 4m. med. Ass., 1964, 189:155-156. 21. SPIRO, R. H., BROCKUNIER, A., and BRUN- SCHWIG, A. Pelvic hemangiopericytoma. Review of the literature and report of a case treated by posterior exenteration. Obstet., Gynec, N. Y., 1964,24:402-410.
22. STOUT, A. P., and MURRAY, M. R. Hemangiopericytoma: a vascular tumor featuring Zimmerman’s pericytes. Ann. Surg., 1942, 116:26-33.
23. TULENKO, J. F. Congenital hemangiopericytoma. Case report. Plast. Reconstr. Surg., 1968, 41:276-277.
24. VIDRINE, A., JR., and WFLSH, R. A. Hemangiopericytoma. 5 cases. Surgery, St. Louis 1964, 56:913-918.
25. ZIMMERMANN, K. W. Der Feinere Eau der Blutcapillaren. Z. Anat. EntwGesh., 1923, 68:29.
